
And elements in group 14 have a charge of -4. The elements in group 13 and group 15 form a cation with a -3 charge each. The main difference between these negatively-charged electrons and cations is that anions do not conduct electricity.

This whole process results in an increased number of electrons with a negative charge. All these elements are grouped in the Periodic Table in the following groups: 13, 14, 15, 16, and 17.Įach of the anions gets its electrons from other atoms as the process of ionic bonding is taking place. What Are Anions?Īnions are formed from all the nonmetal elements. Finally, all the metals in group 14 have a +4 charge. Then, metals in groups thirteen and fifteen have a charge of +3. Group one is composed of metals that have a +1 charge, while all the metals in groups 2,3,4,5,6,7,8,9,10,11,12, and 16 have a charge +2. If you look at the periodic table, you will find the metals in groups (from one to 16). If studying the periodic table taught me nothing else, it’s that the credulity of human beings for periodic table panaceas is pretty much boundless. Electrons carry with them electrical energy when they move between atoms. Any electrons that are lost by atoms that are picked up by neutral atoms will turn those neutral atoms into positive atoms.īecause electrons have such ease of movement between atoms, metals are great electricity conductors. For example, gold, silver copper or sodium. What Are Cations?Ĭations are positively charged atoms that are formed from metal atoms. This then results in the formation of cations (positively-charged ions) and, also, the atoms then pick up electrons from each other, which results in the formation of anions (negatively-charged ions). The process of ion formation involves atoms giving up electrons in order to form other atoms. It is precisely this ability that electrons have to move in orbitals while jumping between different atoms is what contributes to the formation of ions.
#Element ag charge free#
Another thing that makes electrons famous is their free movement around the nucleus in circular directions, making orbital of three dimensions. The isotope of the atom is determined by the number of neutrons and protons therein.Įlectrons are the subatomic particles characterized by their negative charges.

Those particles can be neutrons, which are the neutral subatomic particles located in the very center (nucleus) of the atom together with protons with a positive charge. Ion is the name of the subatomic particles that are components of all the atoms. All the metallic elements located on the left part of the Periodic Table have a positive ionic charge, while all the metallic elements located on the right part of the Periodic Table have a negative ionic charge. There is also a very clear way of knowing whether an element has a positive or a negative ionic charge.
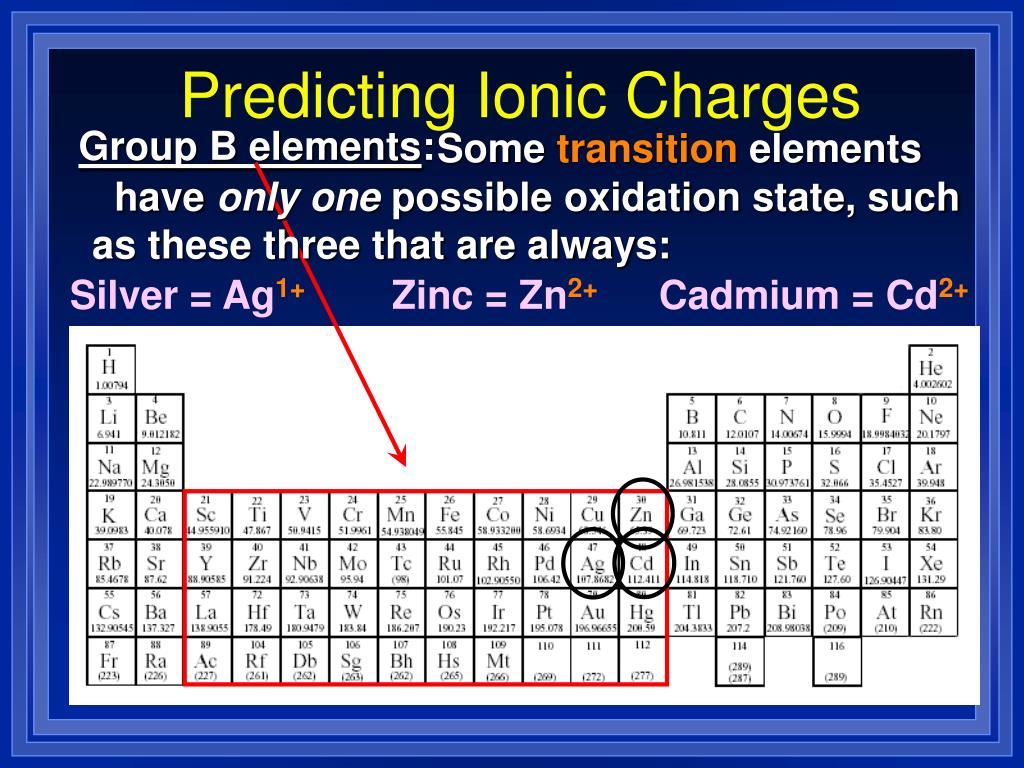
Even a tiny fleck of it stops time.” - Diane Ackerman “Wonder is the heaviest element on the perioid table. The best way to find out what the ionic charge for a specific element is is by checking the Periodic table. The Period Table With Charges is an essential tool for science students. The elements of the Periodic Table have different ionic charges.


 0 kommentar(er)
0 kommentar(er)
